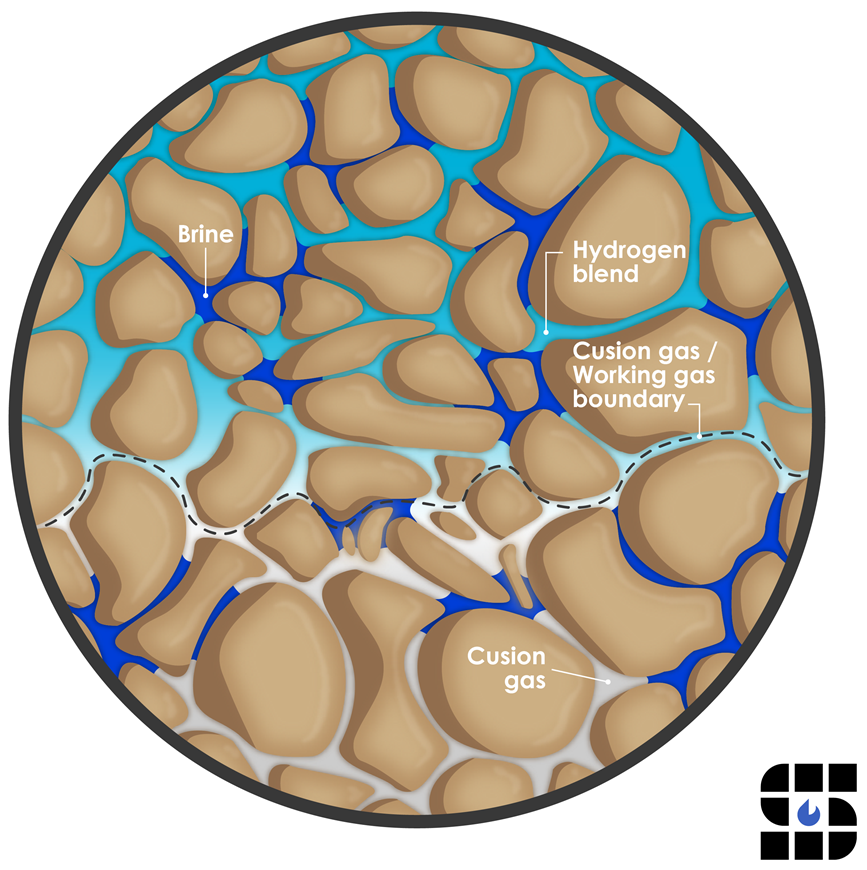
H2 is unlikely to abiotically react with reservoir formations composed primarily of aluminosilicate minerals, though adsorption to some clay minerals is possible. Clay mineralogy is complex and predicting its reactions with H2 is difficult. These reactions, however, tend to cause swelling, which reduces permeability. H2 dissolution into water (e.g., in brines present as thin films on mineral grains or pore throats) can also increase its availability for reaction, and the conditions and extent of these potential reactions are difficult to predict. Secondary reduction-oxidation reactions (e.g., reduction of iron Fe3+ to Fe2+) are the most likely reactions that may alter cementing phases within resistant sandstone reservoirs, as predicted by geochemical modeling and confirmed by recent experimental studies. These reduction reactions can occur on the timescale of years, but their impact on matrix mechanical or permeability properties is uncertain. Other geologic components such as clay minerals, oxides, sulfates, carbonates, and other cement and pore-filling minerals may also be susceptible to reaction with, and function as sinks for (i.e., ‘scavenge’) the dissolved H2.
The impact of H2-brine-reservoir reactions may range from negligible cation exchange with clays, to buffering by carbonates, to dissolution and mineral removal at critical pore throat locations with unexpected changes to bulk permeability. Similarly, the dissolution of minerals within the caprock seal could create new leakage pathways, but research to date indicates that such reactions are unlikely to have a significant impact.
Reactions of H2 with dissolved sulfur species or sulfur-bearing minerals (e.g., pyrite FeS2) can also occur, resulting in mineral dissolution that may alter porosity, permeability, and mechanical properties. Significantly, these reactions may also generate H2S, which decreases the quality of stored H2 gas. Generated H2S can modify both redox potential and pH of resident fluids, which may lead to follow-on fluid-rock reactions. It can also compromise well-field infrastructure due to its flammable, corrosive, and toxic nature. In general, there is not a consensus on the significance of geochemical reactions under storage conditions relevant to UHS.
Geochemical reactions between H2, formation fluids, and rock mineralogy could lead to:
- Loss of H2.
- Contamination of stored H2 by production of other gases (e.g., H2S).
- Mineral dissolution/precipitation, leading to increased/reduced injectivity.
- Mineral dissolution, possibly leading to creation of migration pathways through caprock.
- Mineral dissolution, affecting mechanical properties of the reservoir and caprock.
Any of these changes could compromise H2 storage security and the efficiency of UHS.