As a primary pathway between the surface and the subsurface (underground storage complex), well integrity will be an important source of risk and liability. Wells must endure the stresses created by site operations and always maintain their structural integrity. Wells that lose control of injected or extracted fluids may become a human health or environmental hazard if the injectate is leaked into the atmosphere or groundwater in significant quantities. Well leakage is particularly concerning for H2 storage operations because of the possible hazards created by large quantities of H2 gas. This includes both injection wells and existing wells such as abandoned oil and gas wells.
Maintaining the integrity of wells at underground H2 storage sites may be uniquely challenging because of the subsurface environment created by injection operations. On top of the physical stresses created by injection/withdrawal cycles, these wells must also endure the constant chemical stress created by the H2/CH4 mixture.
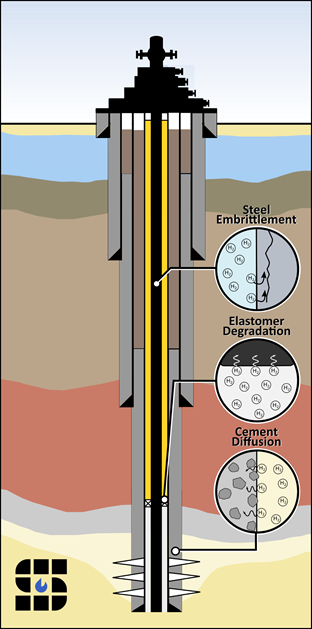
Over the past 20 years, a number of notable well-leakage incidents have occurred at UGS facilities. Loss of containment has typically been caused by failure or deterioration of one or more well component. During construction, a borehole is drilled and lined with steel casing, which is cemented in place. The primary materials of well construction include steel (carbon and/or stainless), polymer elastomers, and cement. It is important to understand how H2 storage operations can impact the integrity of each of these three materials.
The compatibility of H2 with steel and other metals has been extensively documented in the nuclear waste storage literature as well as for applications within the H2 fuel economy. H2 embrittlement, a complex phenomenon wherein H2 moves into the atomic structure of steel, causing premature cracking and failure, is the primary reaction of concern and cause for incompatibility and service degradation. H2 embrittlement can occur when H2 concentration is high enough to permit diffusion into metallic components. This process is distinguished from other corrosive reactions that take place in aqueous fluids. H2-consuming microorganisms have been implicated in causing corrosion, as well as various classes of heterotrophic microorganisms, at temperatures up to 90 °C and high salinities. Rates of microbially-induced steel corrosion in natural and engineered anoxic environments can range from 0.03 to 0.75 mm/yr.
The main challenge for the integrity of wellbore cement is the prevention of gas leakage through the cement. It is believed that diffusivity, rather than reactivity, in cement will be the challenge to effectively store H2 underground. H2 has the highest diffusion rate of all gases due to the fact that it is the smallest molecule in the universe. Currently, there is little data available in the literature of H2 diffusion through cement.
Research has suggested that the most common failure mechanisms in elastomers operating in downhole environments are rapid gas decompression, temperature and chemical degradation, extrusion and nibbling, compression set, wear, and spiral failure. Transport properties of H2 gas in elastomers can vary depending on the operating temperature, as polymers are more sensitive to temperature change than metals. In parallel with experimental work, computational studies have advanced dramatically to offer increased capability of estimating performance of elastomer materials in high-pressure gaseous H2.